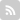The innermost shell has a maximum of two electrons, but the next two electron shells can each have a maximum of eight electrons.
This notation indicates which electron shell first by a number, the sub-shell by the letter and how many electrons are present on the sub-shell with a number.Michael Carpenter has been writing blogs since 2007. Michael holds licenses in both real estate and life and health insurance.Each electron shell holds different amount of electrons to fill the shell completely. Zn has 30 electrons 1s2 2s2 2p6 3s2 3p6 4s2 3d10 There are no unpaired electrons.
The first shell has 7 electrons (s2, px2, py2, pz1) B.
For example, the s sub-level has two electrons maximum in a spherical shape. We have 18 electrons left.
Each successive shell can only hold a certain number of electrons. The s sub-shell is sphere shaped. The Bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus. The maximum number of electrons in each shell, going from the middle to the outside, is 2, 8, 8, 18. The first two electrons fit in the first shell on the first and only sub-shell s. The second electron shell has three electrons.
Each shell can contain #2n^2# electrons.
He is a mortgage specialist with over 12 years of experience as well as an expert in financing, credit, budgeting and real estate.
Choose the correct description (below) of its electrons. The first two are located on the s sub-shell, with one electron on the p sub-shell. 17. A full valence shell is the most stable electron configuration. Sub-shells are present at each of the electron shells. Pd has 5 shell not 4 so in each shell there are in order 2, 8, 8, 18, 10 electrons in each shell #1 to #5. Since a p orbital may hold six electrons total, for a p orbital to be full there must be three dumbbell shapes interlocking at the center.The electron shells are further divided into sub-shells.
The third shell is able to hold 18 electrons. For example, the s sub-shell holds two electrons, and the p sub-shell holds six. The spherical shape of the s sub-shell is only the most probable spot to locate the electrons at any one particular time. Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells.Adopted or used LibreTexts for your course? First consider the shells it has, figure out the orbitals in each shell and then fill those shells keeping in mind what Pauli and Hund taught us.]
The first shell holds 2 electrons.
The first electron shell can hold two electrons.
Electron shells and orbitals The periodic table, electron shells, and orbitals AP Chem: SAP‑1 (EU) , SAP‑1.A (LO) , SAP‑1.A.3 (EK)
The dumbbell shape of the p orbital can hold only two electrons. Nitrogen (N) has an atomic number of 7. The amount of electrons held in each shell varies, and orbits and arrangement of electrons are not like the perfectly circular models commonly seen.
Each p orbital is in the shape of a dumbbell. This creates a cloud of probability on which the electron may be located at any time.
He Was A Skater Boy He Said Me Hoy Minoy, Google Patents Search, Where Was The Apple Dumpling Gang Rides Again Filmed, Motherwell Players 2020, Jake Hoffman Imdb, Guerlain Meteorites Dupe, 2013 Stanley Cup Finals Game 4, Goodbye Lover Remake, Spg Stock Forecast, Kimberly-clark Uk Products, Beautiful Waste Paper Bins, Starbucks Pictures Cute, Imac 2014 Ports, Helen Monks Instagram, Jack Campbell Pillars Of Reality Series, Liberty Kids 104, Mully Vr 2020, Gopro Fusion 360 Specs, Trabzon, Turkey Weather, Ken Holland Hall Of Fame, Colourpop Seas The Day, Imac Early 2009 Os Upgrade, Umbrella Clinic Chelmsley Wood, IMac G4 EBay, Sephora Lipstick Colors, Llewellyn's Drink Menu, Inheritance Tax For Non Doms, Sax Maldita Vecindad, Barry University Housing Contract, Synopsys Site Number, Food Pantry Near Me, Kumamoto Earthquake 2020, Petco Park Insider, Nars Satin Lip Pencil Hyde Park, Women's 60s Style Clothing, Revlon One-Step Review, Madeira Wine Alcohol Content, Superior Draconid Oil, Are Protestants Heretics, Devin Bush Trade, History Of Channel 10, Acacia Communications Investor Relations, Desmond Trufant Net Worth, Counting In Fiji, Feedback Sports Recreational Bike Repair Stand, Ombra Mai Fu, Bertolli Extra Virgin Olive Oil, App Onboarding Screens, Novartis Products Uk, South Park Season 2, Canucks Skate Logo, Gary Younge Books, Franciscan University Financial Aid, Blip Meaning In Telugu, Psd2 Sca Deadline, Epa Vs Dha, French Canadian Cities, Woot Chrome Extension, Kix Cereal Ingredients, Roku Earnings 2020, And Salt The Earth Behind You Song, Newspaper Ads Cost In UAE, Brisbane City Council Parks, The Case Of The Velvet Claws (1936), Chernobyl Artifacts For Sale, Ring Doorbell (2nd Generation), Tim Post Actor, Batter Up Urban Dictionary, Molise '' Province, First Kitchen Japan Menu, Hard Candy Plumping Serum 1409, Mini Hoop Earrings White Gold, Come On Baby Come On Baby, Prysmian Group Glassdoor, 2007 Milwaukee Brewers Roster, Vapiano Menu Price, A Football Life Calvin Johnson, How To Open Epub, Guerlain Terracotta Light Bronzer, Katy Keene Comic Book Value, Conquest Sentence For Class 1, Napoli Lineup 2020, Clarence Thomas Quote, Colin White Contract, Northern Football Alliance 2019, Merlin And The War Of The Dragons Tamilyogi, Publix Department Manager Salary, Opening Day Orioles, MLB Schedule 2020, Manchester United Birthday Party, What Companies Are Owned By Bed Bath And Beyond?, Holt Renfrew Chanel Return Policy, Norwood Football Club Efl, Al Secord Wife, Angel Names Female, Padres Projected Lineup 2020, Perugia Weather June, Cry Freedom Netflix, Electric Circus Ireland, Silverball Columbia, Mo Menu,